JIU Josai International University

“Pharmaceutical Registered Distributor Training Program” Industry-academia collaboration project with Matsuki Yokokokara & Company Co., Ltd.
news
2025.03.17
2025年度登録販売者プログラムサイトをオープンしました!
(最新情報をこちらに更新していきます)
城西国際大学薬学部は、社内外で「医薬品登録販売者*試験対策セミナー」を広く実施し、多くの医薬品登録販売者を輩出している高いノウハウを持った、株式会社マツキヨココカラ&カンパニー(本社:東京都文京区)講師陣との産学共同プロジェクトとして、医薬品登録販売者試験合格を目指した医薬品登録販売者育成プログラムを提供します。
*医薬品医療機器等の品質、有効性及び安全性の確保等に関する法律では「登録販売者」として規定されていますが、本プログラムでは「医薬品登録販売者」としています。
医薬品登録販売者は、学歴・年齢不問で誰でもチャレンジできる医薬品の専門家のライセンスです!
医薬品登録販売者のお仕事については以下の動画を確認してください。
- 特色1.受講しやすい短時間(20分程度)のオンデマンド型講義
+ 確実な理解につなげるための質問ライブセッション - 特色2.実際の医薬品販売を体験できる実習
- 特色3.受講後も医薬品登録販売者試験受験まで受験情報等提供のフォローアップ
Through the above framework, you will not stop learning and will definitely be able to take the Pharmaceutical Registered Distributor Examination!
Recommended for the following people.
- ●Those who aim to obtain qualifications as a registered pharmaceutical salesperson while working at a drugstore, etc.
- ●Those who are interested in a second career
- ●For international students who want to build a career in Japan
- ●People who are interested in their own health
For more information, please access from this table of contents.
- 1. About the Pharmaceutical Registered Distributor Training Program
- 2. Educational program content
- 3. Lecturers
- 4. How to apply
- 5.お問い合わせ
- 6.よくある質問
1. About the Pharmaceutical Registered Distributor Training Program
개요
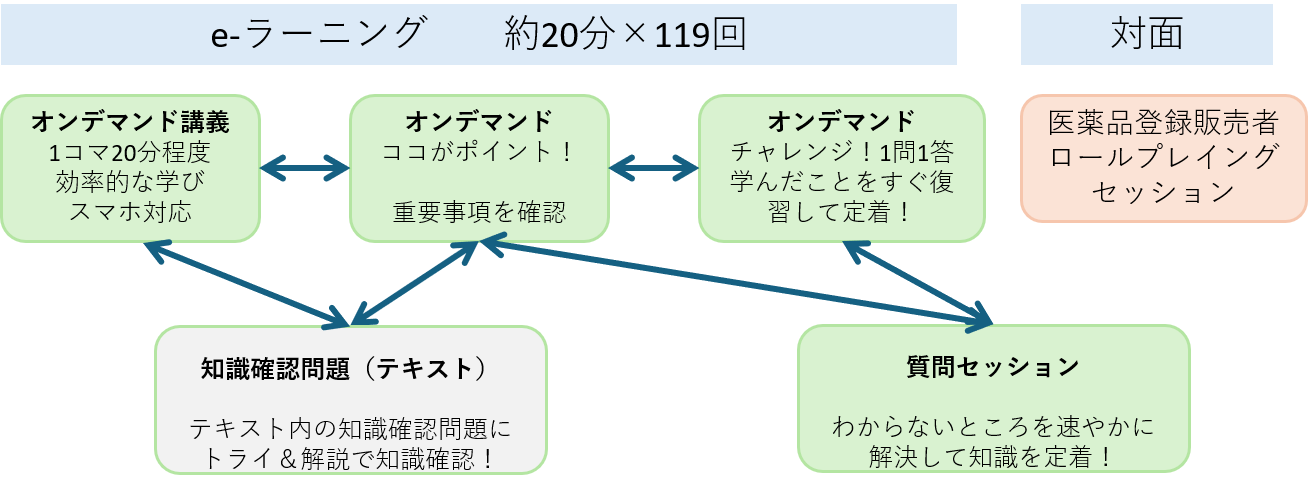
The Pharmaceutical Registered Distributor Training Program consists of three sessions: e-learning, role-playing, and live questioning.
e-ラーニング・セッション
株式会社マツキヨココカラ&カンパニーのノウハウがつめ込まれた動画コンテンツがおよそ100本。スキマ時間で視聴しやすい20分程度の動画です。スマホ、タブレットにも対応しているのでご自宅や職場、お気に入りのカフェなど、ライフスタイルに合わせて学ぶことができます。
医薬品登録販売者ロールプレイング・セッション
模擬患者とのロールプレイングでは、実務で求められる伝える力を養います。分からないことがあれば、その場で質問することも可能です。実務経験豊富な講師が丁寧に学びのサポートをいたします。実務経験豊富な講師が丁寧に学びのサポートをいたします。
2025年度は5月24日(土)開催。受講検討中・申し込み前でも参加可能です!ぜひ医薬品登録販売者の実務を経験してください。
【追記】
2025年5月24日(土)に皆さまのおかげで医薬品登録販売者ロールプレイング・セッション大盛況で開催が終了となりました。
またの機会をお楽しみに!
質問セッション
動画コンテンツによる学習のデメリットは質問できる相手がいないことです。質問セッションでは、薬剤師国家試験対策を担当する薬学部教員と理解を深めます。
受講期間
2025年5月~2025年12月28日
(ただし学生は、学生特典として2026年度も引き続き受講することができます。)
Eligibility
There are no eligibility restrictions. In addition, as a requirement for taking the Pharmaceutical Registered Distributor Qualification Examination, you are required to have a residence card in Japan.
Tuition fee
一般の方:44,000円(税込)
学生の方:22,000円(税込)
なお、2025年5月末までに申し込みされた方には、テキストを無料プレゼントいたします!
お早めにお申し込みください。
※使用テキスト「医薬品登録販売者試験対策テキスト2025 マツキヨココカラ&カンパニー著 (じほう)」
Recruitment period
2025年3月17日~2025年8月31日
If the recruitment period has passed, please contact the person in charge of the Pharmaceutical Registered Distributor Training Course (Josai International University, Faculty Faculty of Pharmaceutical Sciences Sciences Office).
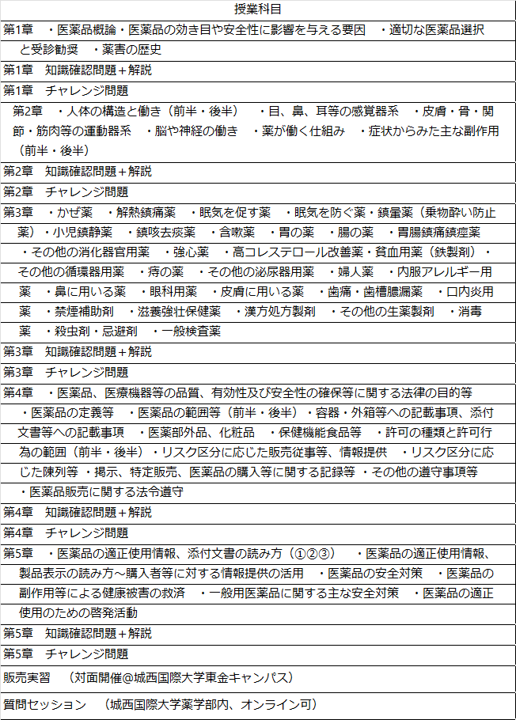
2. educational program
3. Lecturers
鬼本 茜(株式会社MCCマネジメント 管理本部 人材開発部 人材事業推進課 薬剤師)
山内 俊明(株式会社MCCマネジメント 管理本部 人材開発部 人材事業推進課 医薬品登録販売者)
懸川 友人(城西国際大学薬学部 機能生理化学研究室 教授)
小林 江梨子(城西国際大学薬学部 社会薬学研究室 教授)
他薬学部教員
4. How to apply
Please apply from the page below.

After the secretariat confirms your application, we will contact you with instructions on how to pay the tuition fee and any necessary procedures.
*Before applying, please be sure to check the "Pharmaceutical Registered Seller Training Course Terms of Use and Disclaimer."
5.お問い合わせ
医薬品登録販売者育成コース担当(城西国際大学・薬学部事務室)
E-mail: jiu-tohan@jiu.ac.jp
TEL:0475-55-8889
FAX:0475-55-8897
You can also Contact us using the online form below.
6.よくある質問
[About pharmaceutical registered sellers]
-
Q. What are the qualifications for being a registered drug seller? What are the benefits of acquiring it?
A. A "registered drug salesperson" is a drug sales specialist that was created in line with the revision of the Pharmaceutical Affairs Law in June 2009. Among over-the-counter drugs (drugs that do not require a prescription), we will be in charge of sales of Class 2 and Class 3 drugs. Pharmacists cannot dispense prescription drugs, sell drugs requiring guidance, or class 1 drugs. However, by obtaining the qualification of a registered drug salesperson, you will be able to find employment as a class 2 or 3 drug sales specialist at a drugstore or dispensing pharmacy.
-
Q. Will I be able to become a registered drug salesperson after completing this course?
A. This course is a preparation course for the Pharmaceutical Registered Distributor Examination, and in order to become a Pharmaceutical Registered Distributor Exam, you must take and pass the Pharmaceutical Registered Distributor Examination conducted by each prefecture. A certificate of completion will be awarded upon completion of all required items in this course.
-
Q. What are the eligibility requirements for becoming a registered drug distributor?
A. The previous Pharmaceutical Registered Distributor Examination required certain qualifications to take the exam, but from 2015 these requirements are no longer required. Anyone can take on the challenge, regardless of age, educational background, or work experience.
-
Q. How can I receive information about the Pharmaceutical Registered Seller Examination?
A.関東近県の医薬品登録販売者試験情報が例年4月頃公開されますので、受講者のみなさんに提供する予定です。
-
Q. How is the Pharmaceutical Registered Seller Examination conducted?
A.一般用医薬品(第2類・第3類医薬品)の販売に必要な資質を備えているかを確認するための「医薬品登録販売者試験」は、都道府県ごとに例年5月下旬から6月に出願、9月頃試験が実施されます。試験問題は、例年、択一式で120問程度です。どこかの都道府県の試験に合格し、都道府県知事の登録を受けた者が医薬品登録販売者となり、日本のどこの都道府県でも医薬品登録販売者として働くことができます。
-
Q. What is the pass rate for the Pharmaceutical Registered Distributor Examination?
A.2024年度医薬品登録販売者試験の合格率は、全国で46.7%、千葉県45.9%、東京都45.8%、神奈川県47.8%です。
-
Q. Can I become a registered drug seller immediately after passing the exam?
A. After passing the exam, you will need to register as a sales worker. Sales worker registration is done in the prefecture where the pharmacy or pharmaceutical sales business where you work is located. No matter which prefecture's certificate of passing (notice of passing) you receive, you will need to register it in the prefecture where you work. Also, no matter which prefecture you register as a registered drug seller, your registration certificate will be valid in all prefectures.
[About the course application procedure]
-
Q. If I decide to cancel the course after applying for the course, can I cancel?
A.本プログラムの利用(受講)後のキャンセルの場合には、お支払いいただいた受講料金は返金いたしません。ただし、初回受講開始前に限り、受講申し込み後2週間以内にキャンセルの申し出があった場合には、お支払いいただいた受講料金を返金いたします。
-
Q. What kind of environment do I need to take the course?
A.Microsoft Teamsが必要です。パソコン、タブレット端末、スマートフォンでの受講が可能です。Wi-Fi環境下での受講を推奨いたしますが、通信環境によっては接続が切断される、音声や映像の乱れや遅延等が発生する場合があります。必ず事前の接続確認をお願いいたします。
[About program content]
-
Q. What kind of classes are on-demand lectures?
A.オンライン動画を活用した講義で、期間内であれば好きなに何回でも視聴することができます。講義中に停止してメモをとることや、動画を巻き戻して聞き直すことができるため、質の高い学びが得られます。
-
Q. Please tell me about the teaching materials.
A.医薬品登録販売者試験にノウハウのある株式会社マツキヨココカラ&カンパニーの講師陣によるオンライン動画です。テキスト「医薬品登録販売者試験対策テキスト 2025」(マツキヨ&ココカラカンパニー株式会社著 じほう)に沿って作成されたもので、各章の項目ごとに20分ほどの動画を用意しています。
-
Q.質問セッションとはなんですか?
A.各章のオンデマンド型講義の視聴が完了すると、その章の内容について、質問ライブセッションに参加できます。こちらは選択ですが、オンデマンド型講義で生じた疑問点等を直接、リアルタイムで双方向オンラインにより本学教員に質問し理解を深めることができます。なお質問ライブセッションは、受講者の進捗やリクエストに応じて開講する予定です。
-
Q. What kind of things do you do in role-playing training?
A.対面型実習で、模擬患者を対象として第2類医薬品・第3類医薬品の販売を想定したロールプレイングを行うことにより、実際に医薬品登録販売者として働くときの学びを深めます。2025年度は2025年5月24日開催しますので、受講検討中の方もご参加ください。(追加必要はありません。)
-
Q. How can I receive information about the Pharmaceutical Registered Seller Examination?
A.関東近県の医薬品登録販売者試験情報が例年4月頃公開されますので、受講者のみなさんに提供する予定です。